What Best Describes What Happens When a Nail Rusts
What does it mean when there is a physical change. A chemical property describes the ability of a substance to undergo a specific chemical change.
Does Iron Fe Need Water To Rust Is The Presence Of Oxygen Gas Enough Quora
The mass of the rust is equal to the mass of the nail and the combined.
. A physical change b. The mass of the nail is equal to the mass of the rust and the combined oxygen. If we take a particular weight of iron and allow it to rust after complete rusting you will find an increase in weight.
The word equation for rusting is. Occurred when a nail rusts. They were physical changes.
Investigating the rusting oxidation of iron Materials. Through a chemical reaction between iron and oxygen. They were molecular changes.
Through the physical mixing of iron and water. The rusts forms when the iron nail comes in contact with rain and oxygen at the same time which forms rust over time. This actually protects the metal from further corrosion by slowing the rate of oxidation.
Therefore choice 1 is the correct answer because oxidation of iron is a reaction. A reactive change 3. Rusting is the oxidation of metal whereby the oxygen in the environment combines with the metal to form a new compound called a metal oxide.
The mass of the nail is equal to the mass of the rust and the combined oxygen. The mass of the rust is equal to. The mass of the nail is equal to the mass of the rust.
What type of change has occurred when a nail rusts. In the case of iron rusting the new compound is called iron oxide also known as rust. Matter has changed size shape or form.
The Chemical Equation for Rusting. The mass of the rust is equal to one half the mass of the nail. This increase is from the weight of oxygen that reacted and formed rust.
Matter has changed from one substance into another. A chemical change c. Where salt is present electrochemical corrosion occurs and the protective oxide film does not form thus the corrosion buildup of rust continues unchecked.
When an iron nail reacts with oxygen and water in the air it rusts. The mass of the nail is equal to the mass of the rust. How does rust form.
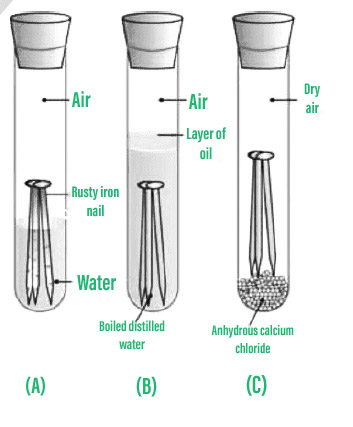
This science experiment is all about controlling variables to explore which material will rust an iron nail first. If the distilled water is allowed to stand in the atmosphere then it may start to rust that nail because of carbonic acid. Acid rain is produced when sulfur dioxide in the atmosphere reacts with water to form sulfuric acid.
A chemical property of iron is that it is capable of combining with oxygen to form iron oxide the chemical name of rust. What happens when a nail rusts. A thin film of oxide forms on the iron.
Put an iron nail in one test tube and label it A and a steel nail in another tube label it B. What is an example of a chemical change that happens inside your body. Matter has changed on the molecular level.
Your iron nail will indeed rust more quickly and. In this worksheet we will practice explaining the conditions necessary for rusting and practice writing balanced equations for the key reactions involved. For more help with your chemistry classes call 646-407-9078 and speak to an amazing Chemistry Tutor in NYC Brooklyn and online.
Is rust bad for skin. Rust is the common name for iron oxideThe most familiar form of rust is the reddish coating that forms flakes on iron and steel Fe 2 O 3 but rust also comes in other colors including yellow brown orange and even greenThe different colors reflect various chemical compositions of rust. Answer The iron in the nail combines with oxygen in the air to form a compound called iron oxide otherwise known as rust.
Given sufficient time any iron mass in the presence of water and. The rusting of iron is characterized by the formation of a layer of a red flaky substance that easily crumbles into a powder. What exactly happens when a nail rusts.
What type of change occurs when iron rusts. The iron in the nail combines with oxygen in the air to form acompound called iron oxide otherwise known as rust. Half fill two test tubes with water.
4 iron nails 1 steel nail a piece of copperbrass 5 test tubes cotton wool solid calcium chloride magnesium ribbon Method. What is the name of the most abundant oxide produced by the rusting of iron. The rusting of iron takes place in the presence of water and oxygen and leads to the compound iron oxide.
When an iron nail reacts with oxygen and water in the air it rusts. A new substance has been formed. Carbon dioxide dissolved in dissolved water produces bicarbonic acid H2CO3 which is an electrolyte.
The experiment in the diagram shows that both oxygen and water are needed for rusting to happen. This rust is formed from a redox reaction between oxygen and iron in an environment containing water such as air containing high levels of moisture. Rust is an iron oxide a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moistureRust consists of hydrous ironIII oxides Fe 2 O 3 nH 2 O and ironIII oxide-hydroxide FeOOH FeOH 3 and is typically associated with the corrosion of refined iron.
Through paint flaking off a metal surface. Rusting is when iron reacts with oxygen to form iron oxides. Which statement best describes what happens when a nail rusts.
An atomic change d. The mass of the rust is equal to one-half the mass of the nail. Which term best describes the two substances that react to form salt.
They were changes in weather. When a nail rusts it is because the nails are made of iron. What happens then to the total mass of the nail.
Calcium chloride in the right-hand test tube. However the rusting will be very slow if the only impurity in the water is carbon dioxide. Choice 2 is also not a chemical property since it describes the physical state of iron.
Rusting is an example of a chemical change. They were chemical changes.
Rusting Of Iron Explanation Chemical Reaction Prevention Geeksforgeeks

Matte Rust Nails Nail Art Nails Beauty

Velvet Rust Nails Choose Shape Finish Set Of 20 False Etsy Bright Summer Acrylic Nails Red Acrylic Nails Baby Blue Acrylic Nails

Pin By Precious Revadonia On My Saves Beige Nails Nail Colors Nails

Flood Science Streamflow Routingstreamflow Routing Describes The Movement Of Water Volume From One Point To Anothe Flood Prevention Flood Preparedness Flood

Lesson Explainer Rusting Nagwa
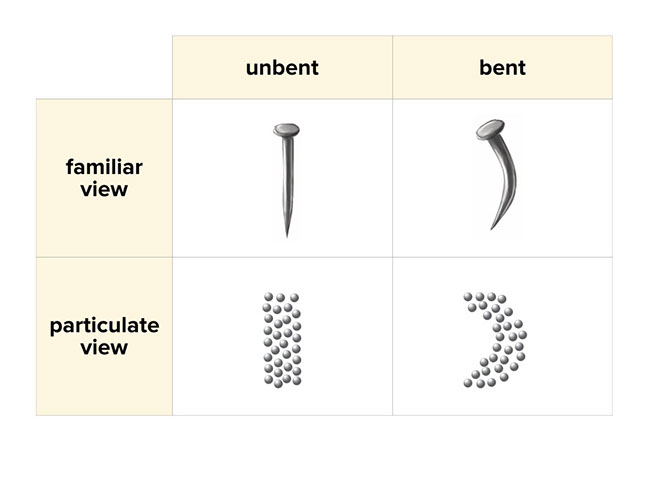
Conversation Describing Physical And Chemical Change

Nail Head Rusting Problems Dulux

Caroline On Instagram Essie Rust Worthy From The Recent Sweater Weather Collection For Fall Essie Describes It As A Warm Nails Winter Nails Winter Nail Art

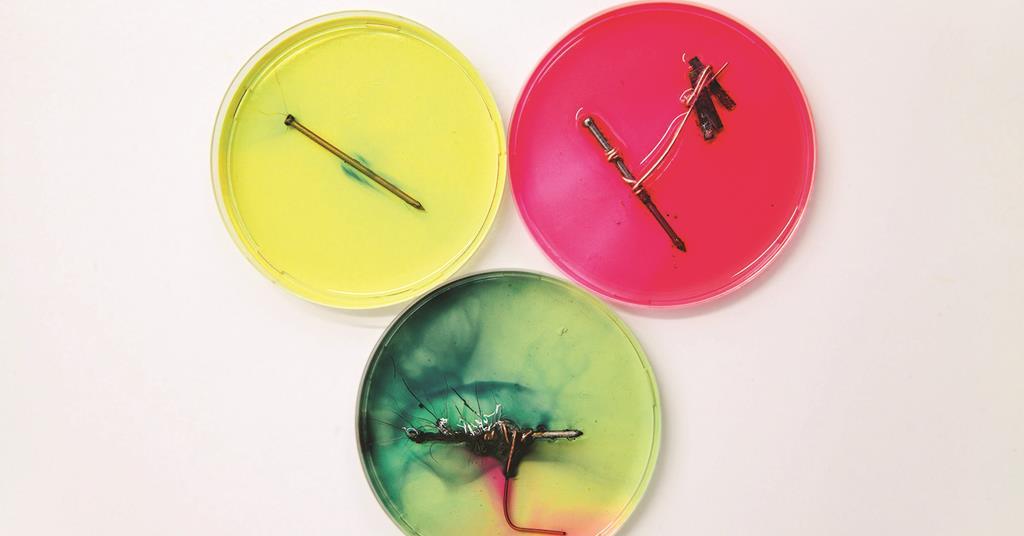
Nailing Corrosion Demonstrations Exhibition Chemistry Rsc Education

A Explain Why The Nails Had Not Rusted In Testtubes B And C In Testtube B In Testtube C Chemistry 11898499 Meritnation Com

Comments
Post a Comment